Our current research is focused on the development and application of biochemical, molecular biology, and structural biology techniques to gain insight into how RNA structures within viral RNA genomes facilitate the viral life cycle in host cells. This research addresses crucial knowledge gaps related to the structures and mechanisms of RNA-mediated genome replication and translation in (+)-sense RNA viruses, many of which are responsible for various human diseases and global pandemics. Our laboratory integrates biochemistry, biophysics, and biology, providing our members with an exceptional opportunity to acquire multiple biochemical and biophysical techniques while conducting advanced RNA structural and molecular biology research. The following are the ongoing research projects in our laboratory.
Structural and mechanistic studies of cap-independent genome translation in (+)-strand RNA viruses
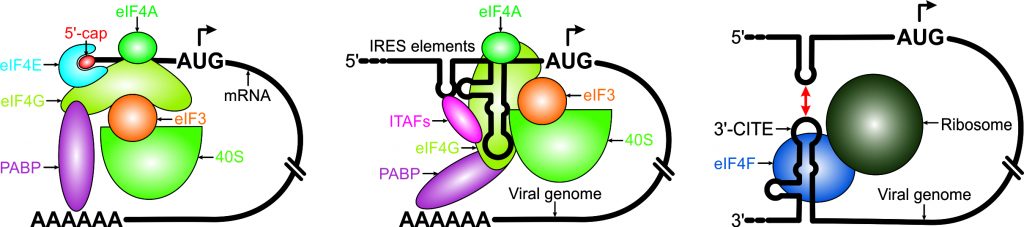 In contrast to the 5′ cap-based canonical translation in most eukaryotes, many viral genomes and a subset of cellular mRNAs are translated using cap-independent mechanisms involving structured RNA elements such as internal ribosome entry sites (IRESs) and 3′ cap-independent translation elements (3′ CITEs). However, the organization and recognition of these RNA structures by eukaryotic translation initiation factors (eIFs) and ribosomal subunits remain poorly understood. This project investigates how IRESs and 3′ CITEs facilitate cap-independent translation in genomes of positive-strand RNA viruses. The findings will offer deeper insights into the mechanisms of cap-independent translation initiation in these viruses and may open new avenues for developing RNA-targeted therapeutics against pathogens causing human, animal, and plant diseases.
In contrast to the 5′ cap-based canonical translation in most eukaryotes, many viral genomes and a subset of cellular mRNAs are translated using cap-independent mechanisms involving structured RNA elements such as internal ribosome entry sites (IRESs) and 3′ cap-independent translation elements (3′ CITEs). However, the organization and recognition of these RNA structures by eukaryotic translation initiation factors (eIFs) and ribosomal subunits remain poorly understood. This project investigates how IRESs and 3′ CITEs facilitate cap-independent translation in genomes of positive-strand RNA viruses. The findings will offer deeper insights into the mechanisms of cap-independent translation initiation in these viruses and may open new avenues for developing RNA-targeted therapeutics against pathogens causing human, animal, and plant diseases.
The crystal structure of saguaro cactus virus (SCV) 3′ translational enhancer that mimics mRNA 5′ cap for eIF4E binding (read the full story: https://www.pnas.org/doi/10.1073/pnas.2313677121)
Structural and mechanistic studies of RNA-mediated enteroviral genome replication
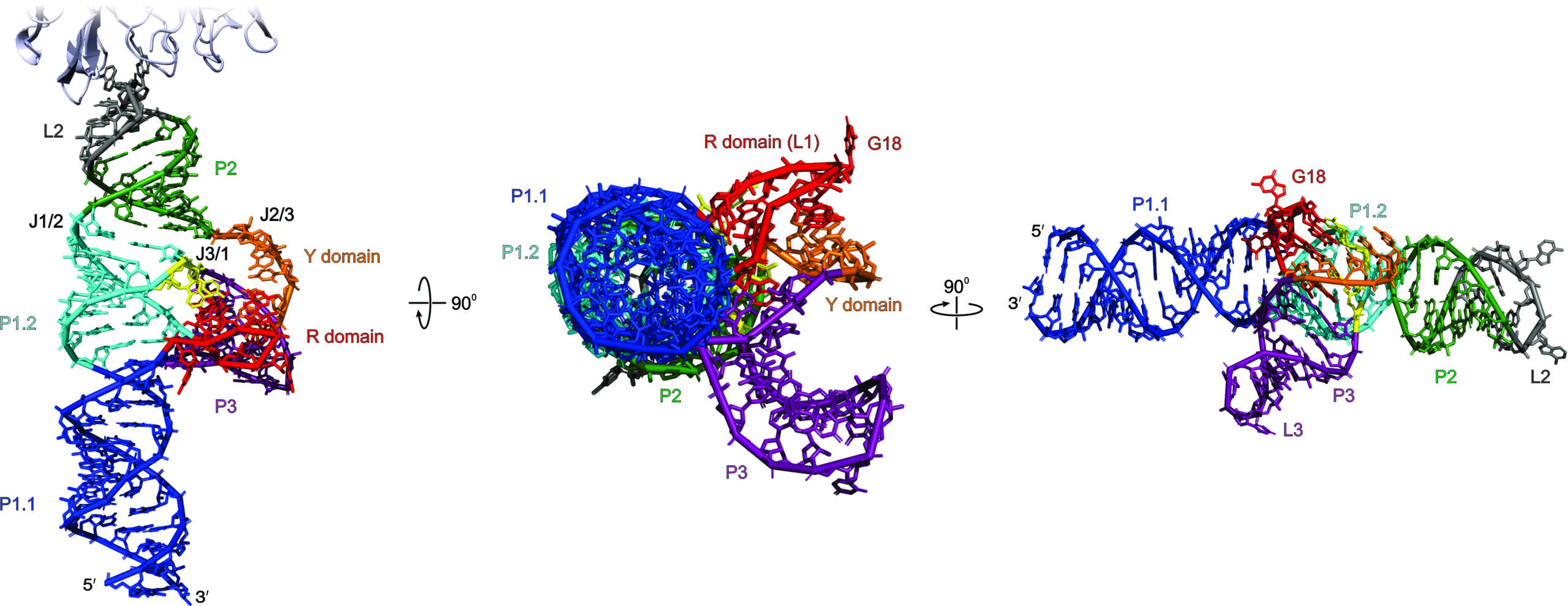
Another major project in our lab focuses on understanding the structural basis of genome replication in enteroviruses. The enterovirus genus of the Picornaviridae family (one of the most prominent families of (+)-strand RNA viruses) comprises a class of pathogens that cause a wide variety of human diseases, ranging from the common cold to poliomyelitis, acute flaccid paralysis, and myocarditis. The genome replication in these viruses, which is essential for viral proliferation, occurs in two distinct steps: the synthesis of (-)-strand RNA using the genomic RNA as the template and then back synthesis of (+)-strand genomic RNA using the newly made (-)-strand RNA as the template. These processes depend on the conserved RNA structures that regulate the viral genome replication by recruiting the viral and the host proteins to form ribonucleoprotein (RNP) complexes and involve these RNPs for virological processes through non-canonical mechanisms. We aim to answer a pressing question regarding the replication in enteroviruses: how does an RNA structure-based strategy promote viral genome replication? Our lab’s approach to answering this question relies on the determination of high-resolution structures of the replication-linked RNAs (REPLRs) found in the viral (+)-strand genomes from seven different species of the enterovirus genus using Fab-chaperone-assisted X-ray crystallography recombinant expression, and purification of viral 3C, 3CD, 3D, 2C, cellular PCBP2, PABP, hnRNP C proteins; biochemical, biophysical, and structural characterization of the REPLR interactions with these viral and cellular proteins; and finally, determination of high-resolution structures of the REPLRs domains found in viral (-)-strands and RNP complexes form the same seven enteroviral species.
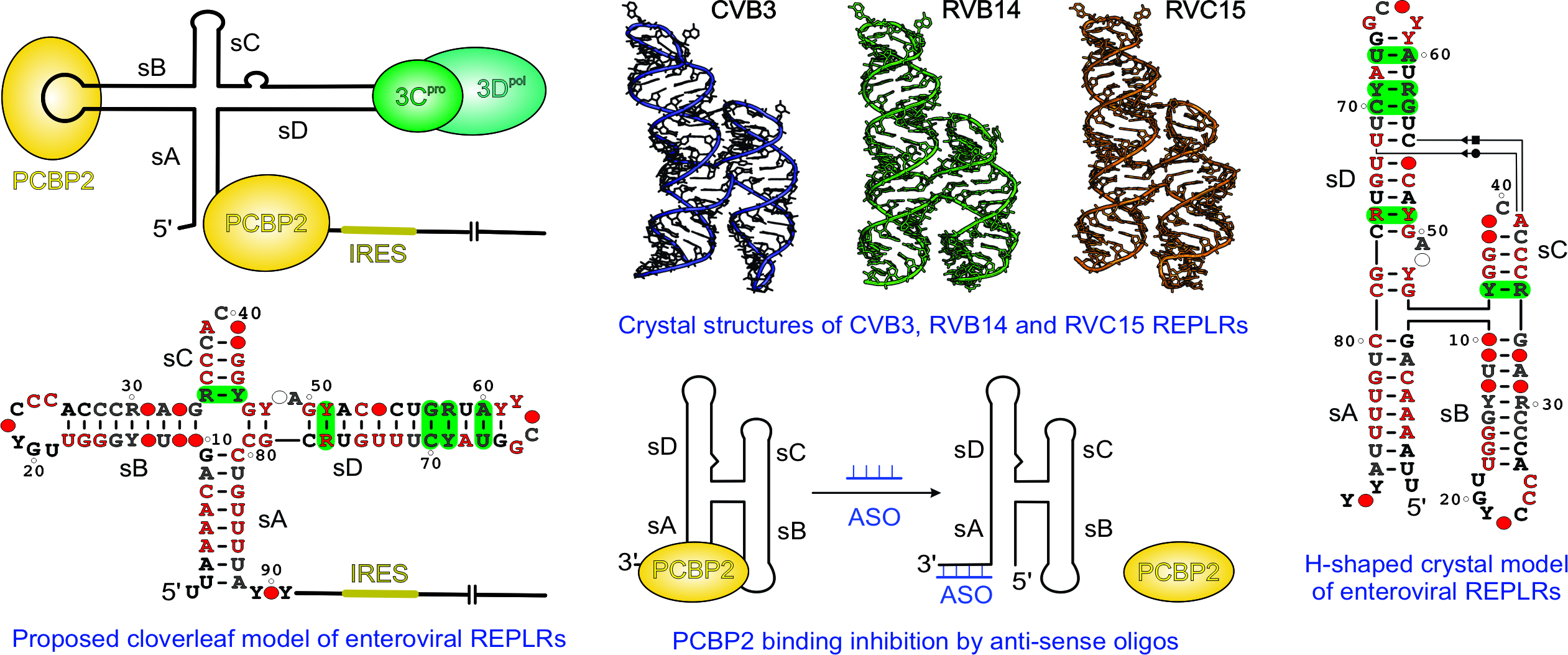
Structural basis of RNA-mediated enteroviral genome replication (read the full stories: https://www.nature.com/articles/s41467-023-37658-8; https://doi.org/10.1093/nar/gkae627)
Structural studies of HIV-1 RRE and FSE
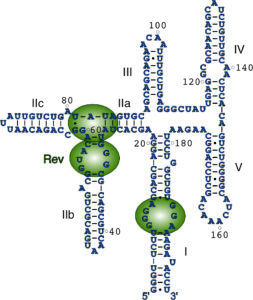
The main objective of this project is to gain insights into the structural mechanisms underlying the nuclear export of HIV-1 RNA through the Rev Response Element (RRE) and the regulation of HIV-1 protein expression through the programmed ribosomal Frame-Shifting Element (FSE). To achieve this, our lab has employed Fab-assisted X-ray crystallography to determine high-resolution crystal structures of the HIV-1 RRE and FSE. Combined with other biophysical and biochemical methods, our lab aims to understand how these RNAs interact with their protein partners to form functional ribonucleoprotein (RNP) complexes.
Approaches for developing RNA crystallization chaperone modules and novel RNA imaging tools
Among several biophysical methods, X-ray crystallography has been the most developed and most widely used method for determining the high-resolution structures of biomacromolecules, including RNA molecules. However, RNA structures represent only a tiny fraction of all deposited high-resolution structures in the protein data bank (PDB), underscoring the difficulties associated with RNA crystallography. We have developed RNA binding proteins, specifically synthetic antibodies (Fab fragments), as chaperones for RNA crystallization and structure determination. In addition to selecting RNA target-specific Fabs using phage display selection, we are interested in developing a suite of portable protein-RNA modules using in vitro selection that can be easily engineered into an RNA of interest as affinity tags, which would have applications in RNA crystallography as well as in RNA imaging and immunoprecipitation.
The outcomes of our research will provide insights into the mechanisms of fundamental biological processes and unlock ample opportunities for developing targeted therapeutics to treat genetic and infectious diseases. Moreover, a structural understanding of the landscape of RNA structures and mechanistic insights into how particular structural features enable biological function will be tremendously valuable for developing algorithms to predict new RNA structures using bioinformatics and computational tools.